Clinical Studies
12 Week Clinical Absorption Skin Trial
Year of study
2015
Duration
12 weeks
This study aimed to assess the absorption levels of the key components of Totally Derma® Nutraceutical Drink Supplement formulation – collagen and HA – in the blood and skin, and to evaluate its effect on skin texture following everyday use of the beverage for 12 weeks.
Test product usage and ingredients
One serving (approximately 12g) of the formulation mixed with 250 ml water or juice to be consumed daily, preferably before sleep, contains:
- 10,000 mg Arthred® hydrolysed collagen
- 210mg Synovoderma® HA powder (9%minHA)
- Synergistic co-factor ingredients: vitamin C (ascorbic acid, 250 mg), grape seed extract (160 mg), green tea extract (160 mg), alpha lipoic acid (60 mg), zinc citrate (5 mg), manganese citrate (3 mg), copper bisglycinate (0.33 mg)
Study design and subjects
- This was a 12-week usage study assessing the absorption of daily Totally Derma® Nutraceutical Drink Supplement ingestion, by examining absorption levels of the key ingredients, collagen and hyaluronic acid, in blood serum and skin biopsies.
- Study Population: 6 women aged 46–65 years with mild to moderate general facial lines and coarse wrinkles in the eye area, skin dehydration and mottled pigmentation on the face. Subjects continued their regular skin care regimen alongside consumption of the nutraceutical beverage.
Assessments
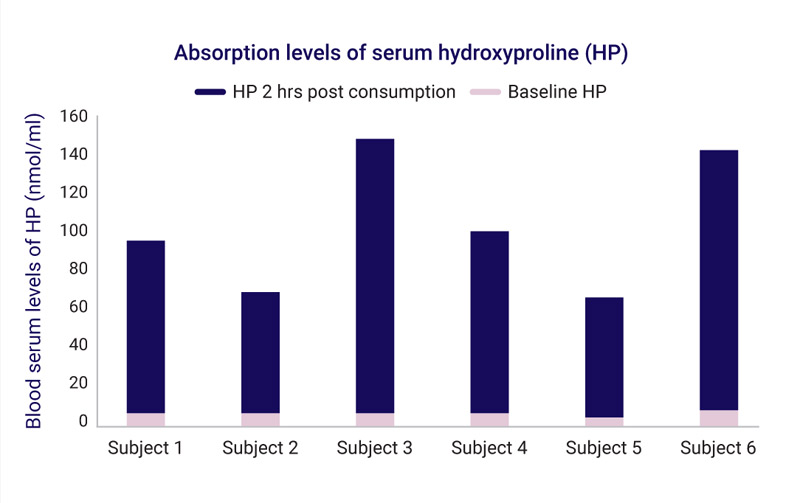
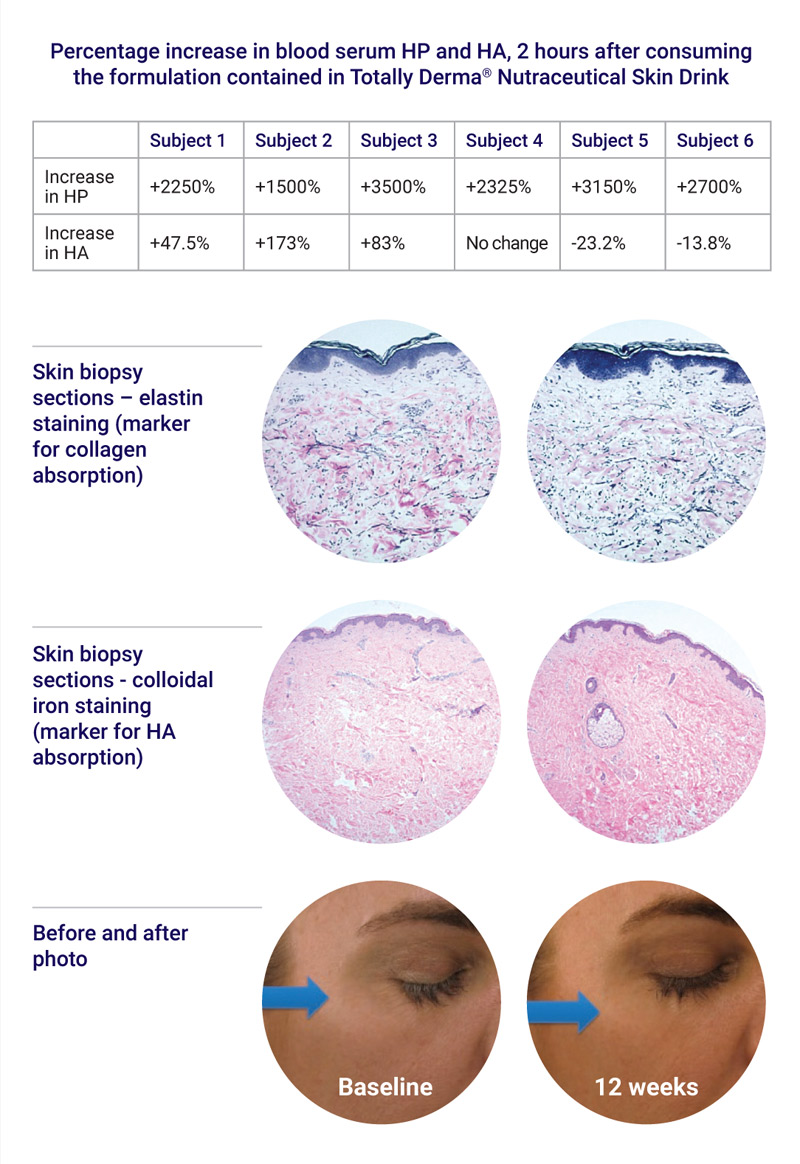
- Blood serum was assessed at baseline (fasting) and 2 hours after beverage consumption for levels of hydroxyproline (as a marker for collagen serum absorption) and HA.
- Skin biopsies from non-sun exposed skin (hip region) were collected at baseline and after 12 weeks of daily use of the drink supplement, and were sectioned and stained for hematoxylin and eosin (H&E, control) and for the following markers:
- Elastin as a marker for collagen absorption
- Colloidal iron as a marker for HA absorption
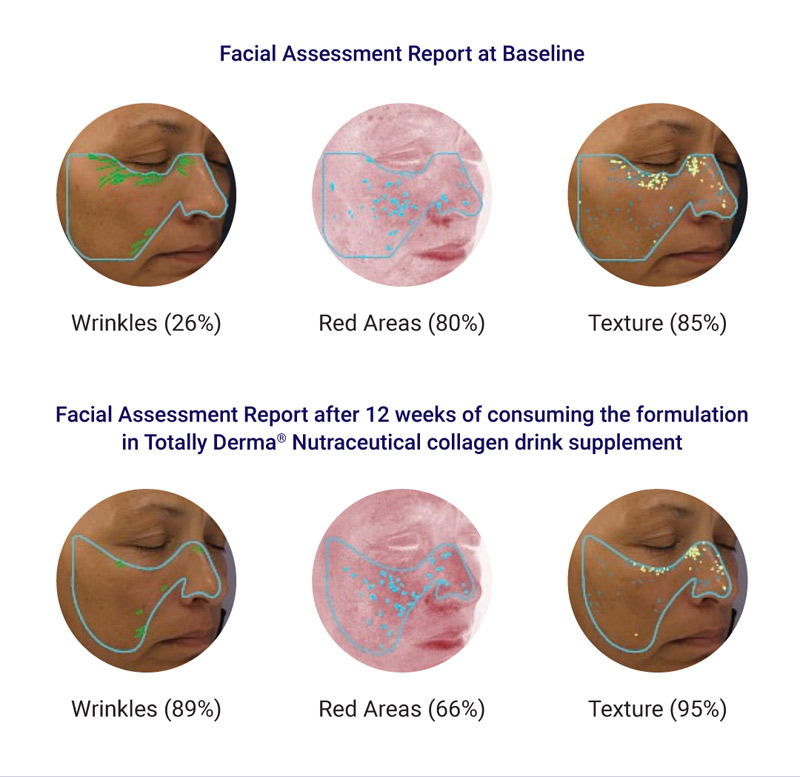
- Visia Skin Analysis Machine facial assessment at baseline and 12 weeks
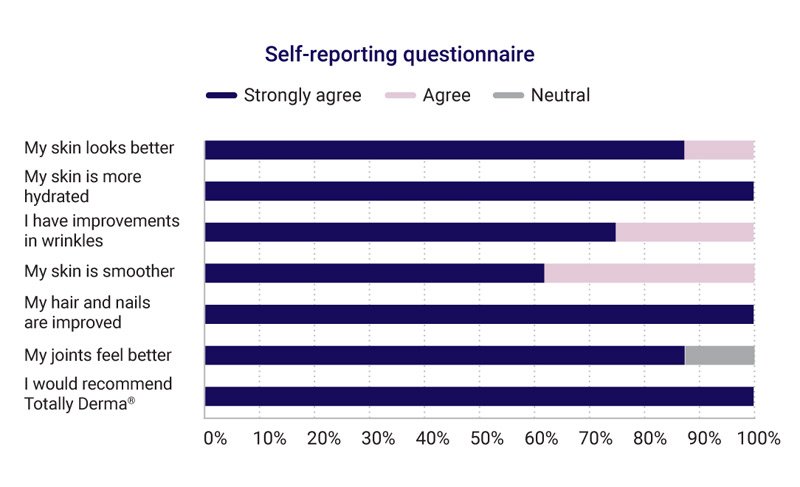
- Retrospective self-reporting questionnaire
This study confirms that the key components of the formulation of Totally Derma® Nutraceutical Drink Supplement, collagen and HA, are being effectively absorbed when ingested and lead to increased levels of collagen and HA in the skin, visible anti-ageing effects and an improved overall skin texture.
The markedly elevated blood serum levels of hydroxyproline following ingestion of the beverage show impressive collagen absorption levels.
The formulation of Totally Derma® Nutraceutical Drink Supplement was well tolerated and highly rated by the study group who all observed improvements in their skin.
This study supports the notion that ingesting specific ingredients can result in an anti-ageing and ‘health-boosting’ effect on the skin. Furthermore, oral nutraceutical supplementation appears promising for skin rejuvenation.
The right balance of specific, optimal quality and high purity ingredients is crucial. The specifi c formulation of Totally Derma® Nutraceutical Drink Supplement works from within to stimulate the body’s own production of collagen, elastin and HA, thus addressing the underlying physiological skin ageing process to help restore the look and feel of youthful skin.
10 Week Clinical Study
Year of study
2015
Duration
10 weeks
To evaluate the benefits of active ingredients offering excellent solubility and can be digested and absorbed in the small intestine; before being distributed through the blood vessels through the deeper layer of the dermis, resulting in the multiplication and stimulation of fibroblast cells to produce more collagen and hyaluronic acid in the dermis.
Design: Visia Skin Study Analysis
Population/Inclusion: Caucasian women, aged 47-56 with mild to moderate general facial fine lines and coarse wrinkles in the eye area, skin dehydration and mottled pigmentation on the face and willing to try an ingested high strength collagen drink supplement.
Exclusion: Current use of skincare includes routine use of anti- ageing topical lotions and creams. No cosmetic procedures (Botox, fillers, peels, laser, light treatments) used by any of the candidates.
Duration: 10-week study (March-June 2014) Evaluation Visits: Baseline, at 6 weeks, at 10 weeks Evaluation Tools: Visia Skin Analysis machine Photography: Baseline, at 6 weeks, at 10 weeks.
Key ingredient benefits
10,000 mg. Arthred® (95% absorbed) highly bioavailable enzymatically processed hydrolysed Collagen peptides raise Hydroxyproline amino acids (the main building block component of collagen and elastin) by 550%.
210 mg. enzymatically processed Hyaluronic Acid increases skin hydration, volume and density and supports collagen and elastin.
Synergistic co-factor ingredients stimulate a natural biological process which promotes the stages of collagen synthesis, maintenance, promotion and repair.
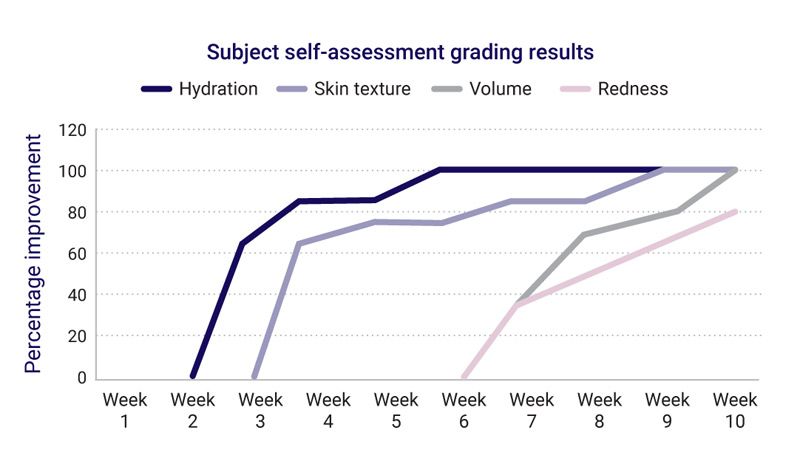
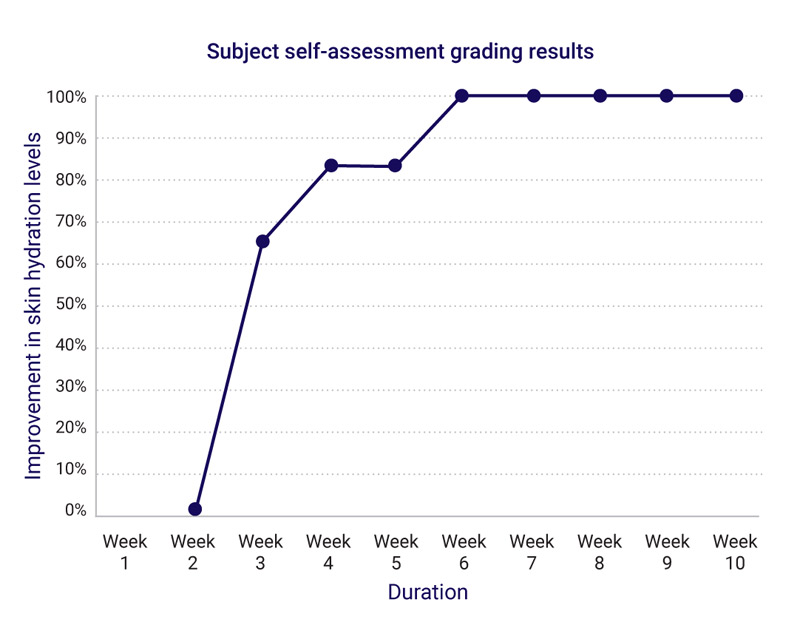
Week 3 for skin hydration
Week 4 for more even complexion, glowing skin, overall appearance
Week 5 for less redness and spots, dewy hydration and clarity week 6 for improved skin smoothness, density
Week 7 for wrinkles and visual roughness
Week 8 for crows feet improvement
Week 10 for all the above
Clinical skin study photography
Drag the arrows to compare
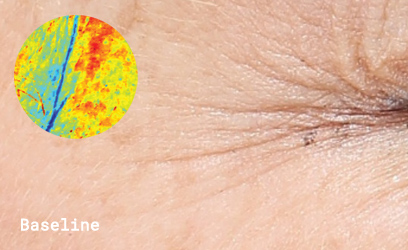
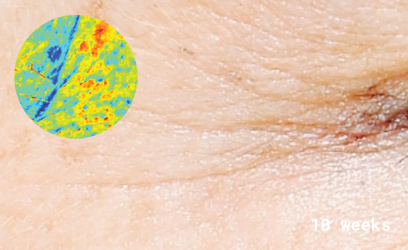
Drag the arrows to compare
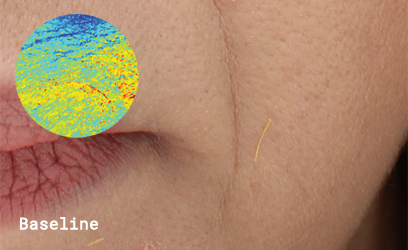
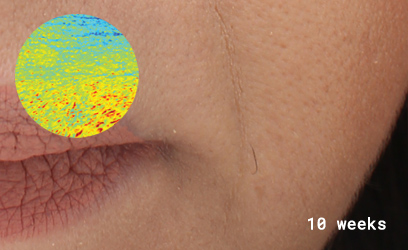
After 10 weeks, group participants agreed:
100% skin was more hydrated, texture was smoother, firmer, had more volume, wrinkles less noticeable.
80% less redness.
Formulation delivers advanced comprehensive anti-ageing benefits to dehydrated, lax, wrinkled, sallow skin through the synergistic actions of multiple benefit ingredients, is 95% absorbed and is well tolerated.
These anti-ageing effects are clinically measurable and statistically significant in working from ‘within’ to stimulate the body’s own production of collagen, elastin and HA, addressing the signs of the ageing process to help restore the look and feel of youthful skin.
Smoother and more hydrated skin
Improvement in wrinkles
Hair and nail improvement
My joints feel better
3 Month Cystic Acne Skin Trial Study
(Gut Health)
Year of study
2021
Duration
3 months
The bacteria present in our gut have an enormous effect on our overall health and well-being, dictating many internal processes in our bodies. Poor gut health, including changes in the gut microbiome and permeability in the gut lining, allows particles and toxins to pass through the digestive tract into the bloodstream, where they can kick off an inflammatory cascade, known as a leaky gut syndrome. This inflammatory response can then be communicated to the skin, triggering cystic acne, dermatitis, rosacea, and other skin conditions.
As more and more research is emerging in support of the importance of the gut-skin connection for skin health, we were inspired to conduct a trial of our own and see if ingesting Totally Derma® could improve cystic acne.
This trial aimed to assess the benefit of ingesting nutraceutical collagen drink Totally Derma® for treatment of cystic acne following everyday use of the supplement for 12 weeks.
The patient (female, age 25) began to develop mild acne in the late summer of 2020, which worsened and developed into cystic acne in a matter of a few weeks. The patient started a skincare regimen, comprised of topical products described below 2 months prior to the trial with no improvements.
A 90-day supply of Totally Derma® was provided to the skin trial candidate. She was informed and understood the importance of compliance in drinking Totally Derma® daily.
The patient maintained her consistent skincare routine throughout the 3-month trial. For clinical research and data purposes, the candidate agreed to keep a journal of her weekly journey and submit 2 before photos (left and right side of the face) and subsequent weekly photos over the 12-week trial.
During the 3-month trial, the patient was following a skincare regimen consisting of 3 prescription topical products:
- Dermol 500 lotion
- Erythromycin (zineryt) lotion
- Benzoyl peroxide 5%
The candidate was not taking any oral medication throughout the trial. Neither could she undergo any clinical treatments due to severity of skin condition (microneedling, microdermabrasion, laser, etc.)
Drag the arrows to compare
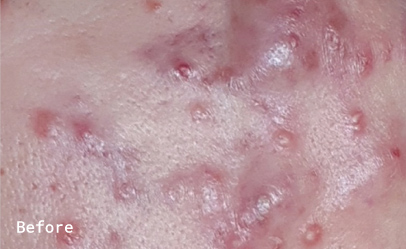
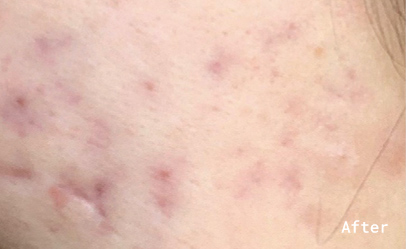
The patient noticed a huge improvement in the condition of her skin within the first 2 weeks of taking the supplement. She reported that swelling around the inflamed areas on her face had gone down and the cysts had flattened. In weeks 3 and 4, the candidate suffered from a cold, which triggered a worsening in her skin condition and she also skipped the supplement a few nights in a row. By the midpoint of the trial, a major difference can be seen between the baseline photos and photos taken during week 6 of ingesting Totally Derma®. The patient reported that “it is the best my skin has looked in a very long time”.
In weeks 7 and 8 the candidate reported further clearing of the skin, followed by minor breakouts in weeks 9 and 10, which coincided with the luteal phase of her menstrual cycle – a phase that is often associated with the increased sebum production. By the end of the 3 months, the patient’s skin had improved significantly and no active breakouts or cysts were reported.
The patient was very satisfied with her skin condition and her only concern was the post-acne scarring. The candidate reported that her skin remained clear for 2 months following the trial but 3 months after stopping Totally Derma® her acne has returned, however not nearly as aggressive as before the trial.
The benefits of Arthred® the main ingredient in Totally Derma®
(Joint Health)
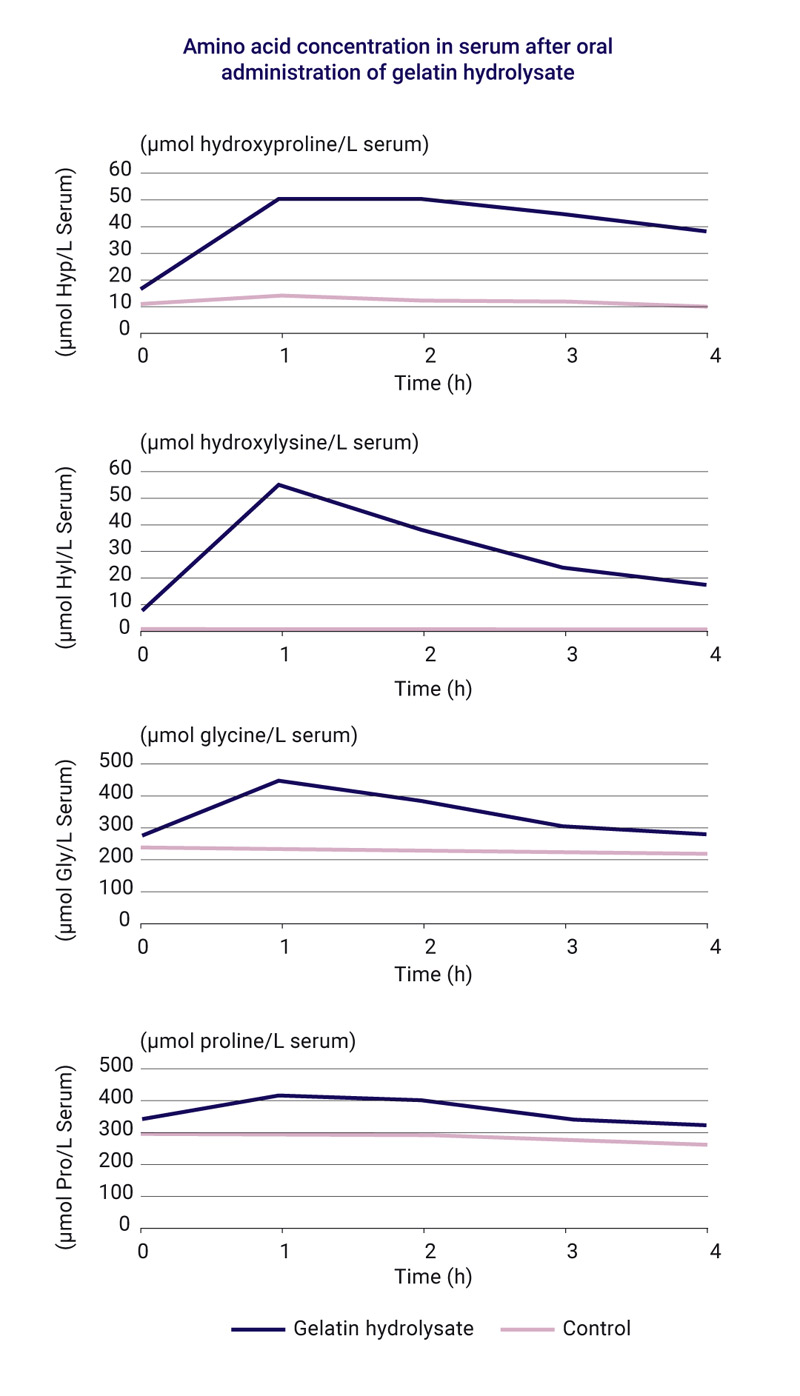
Hydroxyproline is one of the main components of Arthred™ hydrolysed collagen and is therefore a good marker whose uptake can be quantified in blood serum (10). The absorption of short-term orally administered hydrolysed collagen was measured by a single supplementation of 10g Arthred™ hydrolysed collagen. When compared to a standard diet control without hydrolysed collagen, supplementation resulted in a significant increase in collagen- characteristic amino acids like hydroxyproline, hydroxylysine, glycine, and proline in blood serum [9] (see below).
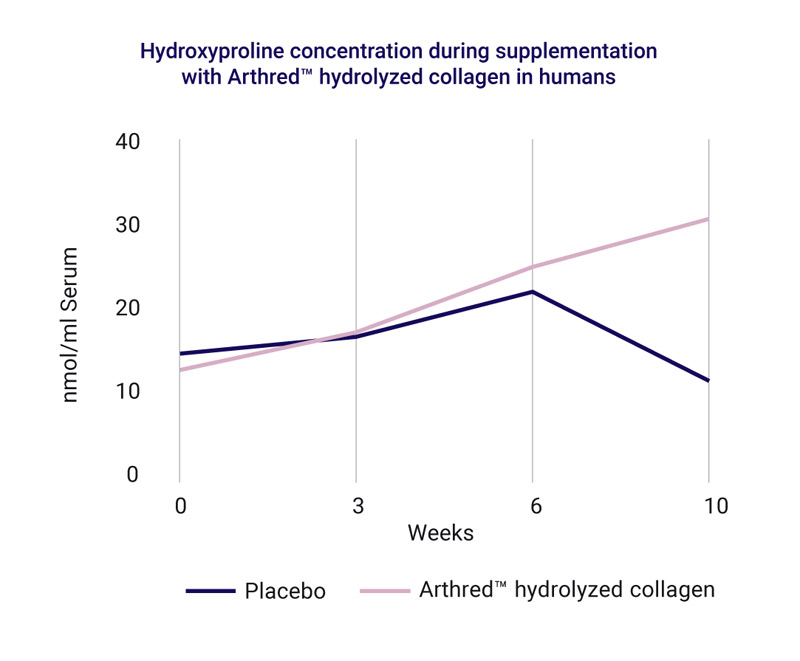
Beuker et al. (1993) conducted a double-blind, placebo-controlled clinical trial involving 52 student athletes (aged 21 to 27 years) to investigate amino acid concentrations in serum following long-term oral administration of Arthred™ hydrolysed collagen or a standard diet without hydrolysed collagen (placebo) during exercise [10]. The athletes performed one hour of physical training three times a week during a period of four months. The collagen-specific amino acid hydroxyproline concentration in plasma was continuously and significantly elevated during supplementation with hydrolysed collagen providing evidence that collagen was absorbed into the bloodstream. The placebo had no effect on hydroxyproline levels in the blood (see above).
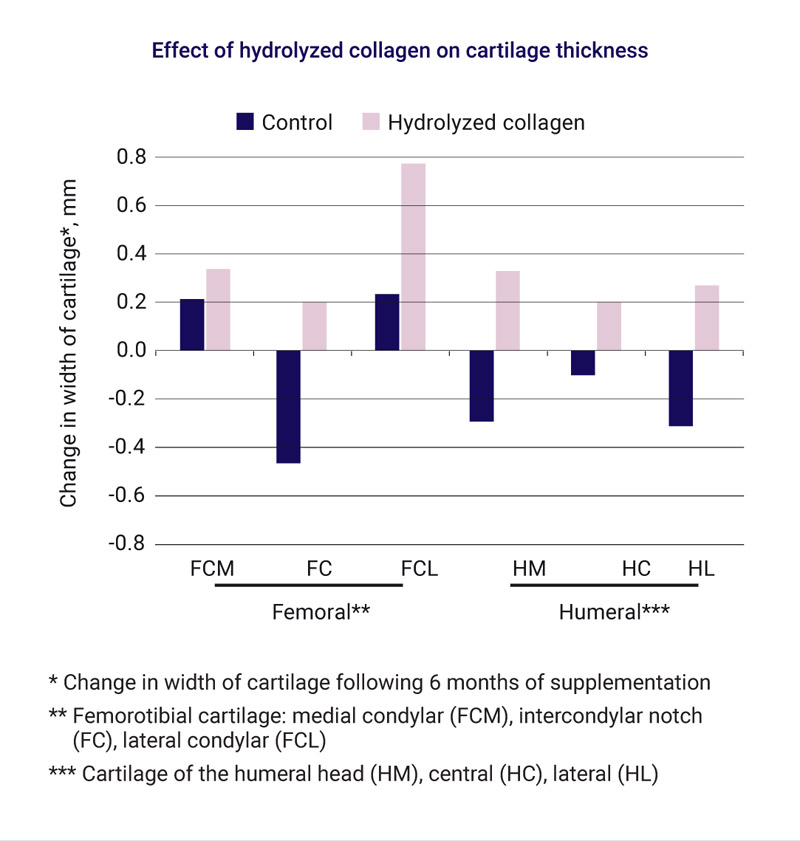
Among healthy, competitive Spanish male mountain bikers (n=16) and female basketball players (n=10), Fernandez and Perez (1998) examined the effect of hydrolysed collagen (also called hydrolysed gelatine) on shoulder and knee joints. Subjects consumed hydrolysed collagen (10g/day) enriched with magnesium, vitamins B1, B2, B6 and pantothenic acid for a six-month period [11]. After supplementation, biometric ultrasound values of the cartilage showed statistically significant increases of cartilage thickness in supplemented athletes while control subjects exhibited either no change or decreasing cartilage thickness (see below). This indicates that enriched hydrolysed collagen may help maintain cartilage homeostasis and joint health among athletes whose joints have been stressed through competitive athletic performance.
3 Month Clinical Trial
Genitourinary Syndrome of Menopause (Gsm)
(Menopausal Health)
Year of study
2021
Duration
3 months
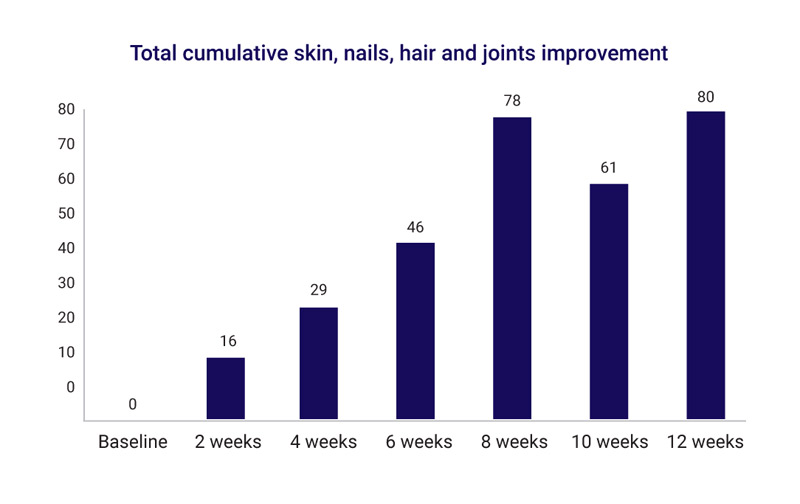
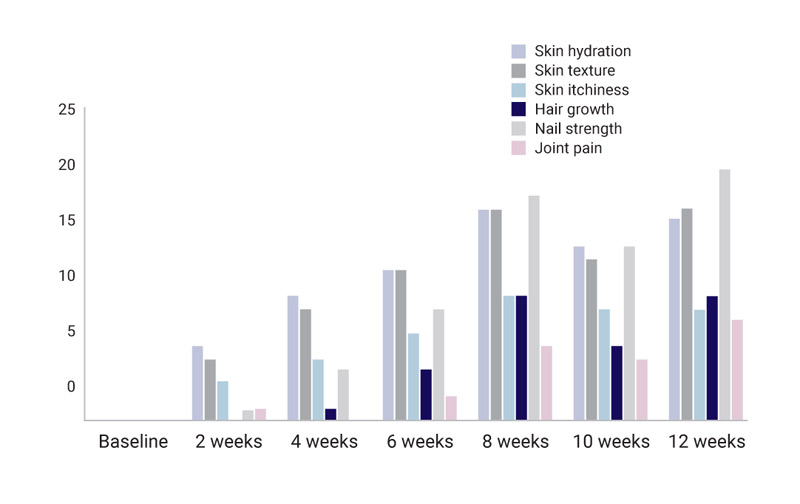
This study is aimed at quantifying anecdotal reports that Totally Derma® has helped women who take it with their GSM symptoms, as well as assess the improvements in skin, nail, hair, and joint health of these women. While it is a small study, a 70% improvement in GSM symptoms has been reported, and almost 90% of participants have seen a boost in their skin, nail, hair, and joint health.
Eight women with an age range of 48-61 were instructed to take Totally Derma®, a hydrolysed bovine collagen supplement*, at night for 12 weeks.
Seven of the eight women had not taken this or other collagen supplement regularly before or within the last 12 months. None had any diagnosis of vaginal or other significant health conditions that would interfere with results or render them ineligible due to contraindications from taking the supplement. Seven of the eight were post-menopause, one was perimenopausal, and the average age was 51. Five were using transdermal or oral HRT, in addition, one was using ovestin, one was using vagirux and one was using a DHEA supplement.
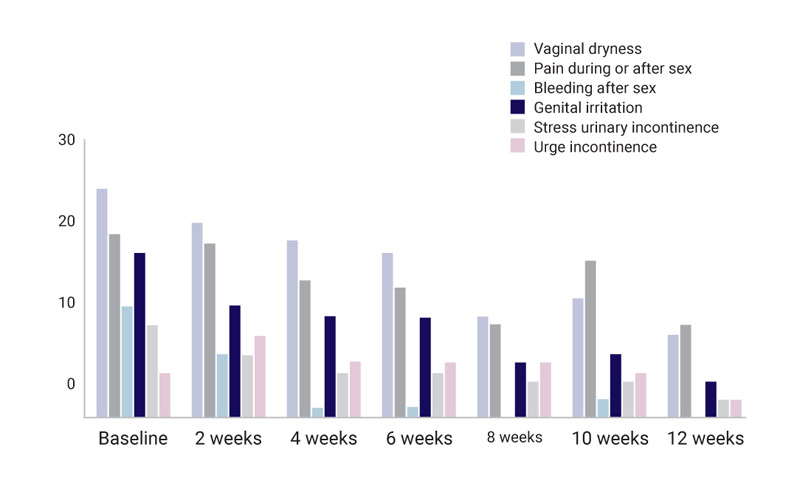
The women were asked to rate their GSM symptoms (vaginal dryness, pain during or after sex, bleeding after sex, stress urinary and urge incontinence, genital irritation) every fortnight for three months with a rating scale of 0-5.
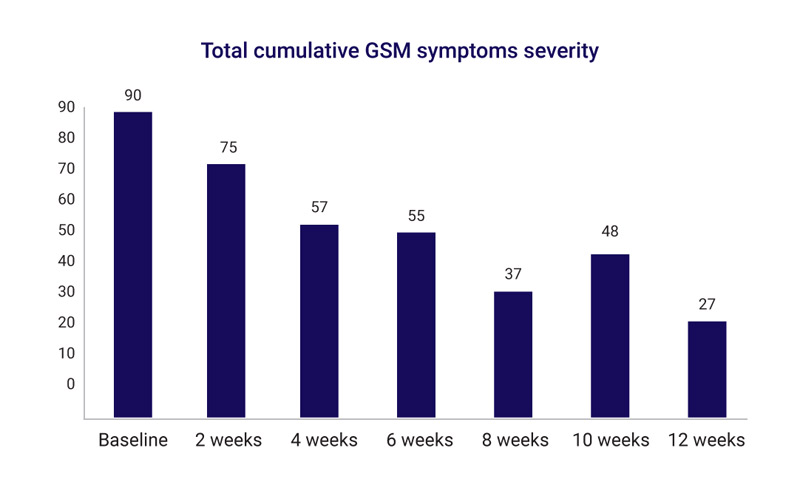
The first report was considered the baseline and was compared to the results after 12 weeks for all symptoms measured.
The first report was considered the baseline and the cumulative score of symptoms severity was 90. This dropped steadily during the course of the study to reach 27 by the end – a 70% improvement in symptoms. Individually there has been an improvement in all symptoms.
Across the board, 88.8% of the participants saw improvements in all areas measured. Skin and nail strength performed the best with a 65% improvement in skin hydration, 71% in skin texture and a 95% improvement in nail strength and appearance reported. Skin itchiness improved by 70%.
A total 70% improvement in all GSM symptoms.
- All women said the bleeding after sex had stopped.
- 80% said the stress urinary incontinence had improved.
- 60% said their urge incontinence had improved.
- 77% said the labial symptoms were no longer as severe, with one experiencing no symptoms at all.
- 64% said their vaginal dryness improved.
- 50% said pain during sex had reduced.
- Two who had not been able to have sex for years were able to.
- One was pain-free.
All women saw improvement in skin hydration, skin texture, and nail strength.
- 62.5% of women saw improvement in hair growth, with 37.5% of women reporting good and moderate improvements.
- 62% reported an improvement in skin itchiness.
- 37.5% of women also saw a good to moderate relief in their joint pain.
While participants were quick to see results with skin hydration and texture with improvements starting within two weeks, nail, hair and joints took longer to advance, as expected. More participants started reporting improvements in those areas after about 6 weeks. One woman reported a month after the trial that she could now see new good hair growth in an area of previous temporal hair loss consistent with hormonal changes and female pattern baldness.
Totally Derma® collagen supplement shows promising results for perimenopausal and postmenopausal women with moderate to severe genitourinary symptoms associated with menopause. Its formulation of enzymatically processed hydrolysed bovine collagen was well tolerated by most and no significant issues were reported. While this is a small, self-reporting study the results are promising with the supplement offering relief for 90% of the participants across the full range of GSM symptoms. The study also indicated that Totally Derma® can be beneficial for skin, hair, nails and joints.